Session II-3. Microbial Process Kinetics
The kinetics of dissolved contaminant biodegradation are discussed in chapter 4 Wiedemeier, section 4.4 pages 178-186. There are three mathematical models used to describe biodegradation kinetics:
- Zero order (i.e. biodegradation rate is constant with time).
- First order (i.e. biodegradation rate is directly proportional to dissolved contaminant concentration).
- Monod kinetics.
These three kinetic expressions are discussed in section 4.4. Assignments using the BIOSCREEN model will provide additional background on biodegradation kinetic concepts. Begin by reading the following 4-page tutorial, entitled “Microbial Kinetics”, which describes how the Monod kinetics growth curve is is developed in well-mixed batch reactors in the laboratory. The relationship between “Monod kinetics”, and “zero” and “first order” kinetics is subsequently explained.
Microbial Kinetics
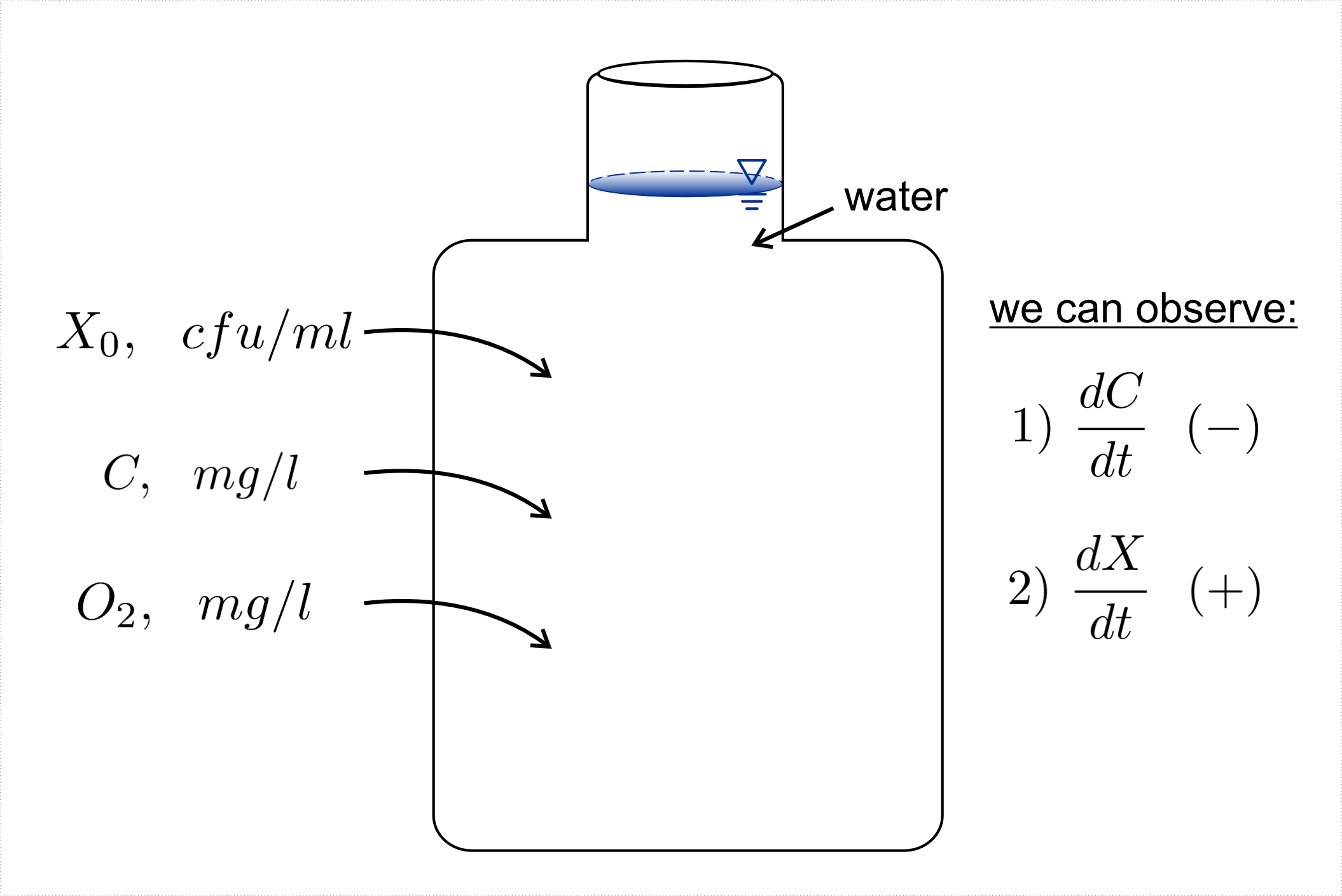
Known concentrations of Oxygen (O2,mg/l), primary growth substrate (C,mg/l), and microbial inoculum
(X0, cfu/l) are added to a well stirred container of water (Figure II-12). Microbial activity begins converting
substrate into more microbial cells (biomass).
With these Observations, we can compute:
1. Yield coefficient * :
S = total Mass of Substrate consumed during interval dt
* Note: Y is sometimes called the 'substrate-to-biomass yield'
2. Specific growth rate:
It follows that:
where “X” is biomass concentration at any arbitary time “t”.
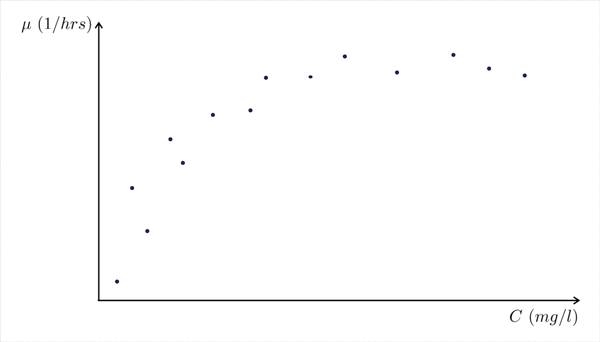
Now, if we repeat this procedure, keeping O2 and X0 constant but changing substrate concentration “C” over a wide range of values, we will generate a “μ” value corresponding to each C value and we can make the following graph (Figure II-13):
Each point on the graph represents the “μ” value observed for a particular substrate concentration in
the flask.
Monod Kinetics
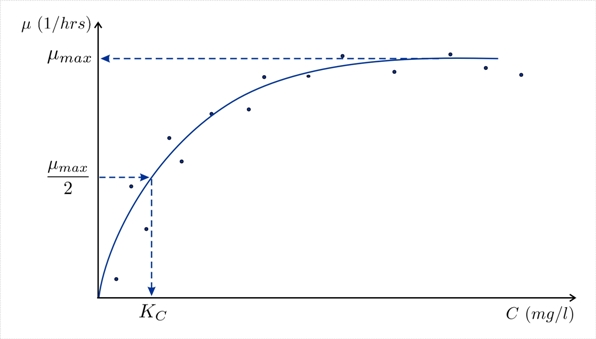
To obtain Monod Kinetic parameters, we first fit the Monod saturation equation through the data in Figure II-13.
This saturation equation is:
From Figure II-14 we see that μmax is equal to the maximum specific growth rate observed. KC is seen to be
the substrate concentration corresponding to
.
Based on the Monod Saturation relationship we have:
Zero Order Kinetics>
From the Monod Equation for Contaminant Biotransformation Rate (and Figure II-13) we see that if C >> KC:
First Order Kinetics
From the Monod Equation for Contaminant Biotransformation Rate (and Figure II-13) we see that if C << KC :
where “Kb ” is defined as the first order biodegradation rate coefficient.
Written Assignment: Questions II-7 through II-11.
Next Session