Model System for Growing a Standard Biofilm: Static Glass Coupon Reactor
Student Version (go to Instructor Version)
Introduction:
The ability to create biofilms with typical characteristics that are consistently reproducible is of great importance to researchers in medicine, industry and academia. The technique described here was devised in an industrial laboratory to create uniform, reproducible biofilms for testing the effectiveness of cleansing and disinfecting products. Prior to the creation of this technique, industries wishing to test their products typically would spray planktonic cells onto sterile glass slides, dry them and then spray them with the selected product. Often the “kill” rate observed in these laboratory tests and the rate observed on naturally occurring microbial populations on surfaces were markedly different. This observation is explained by the unique properties of biofilms that include many phenotypic differences from planktonic cells including resistance to antimicrobic substances.
Supplies Needed:
|
Quantity |
Description |
| 1 | square petri plate (10 x 10 cm) of Trypticase Soy Agar |
| 2 | tubes containing 9 ml of 1/10 strength sterile Trypticase soy broth |
| 1 | piece of sterile Whatman #2 filter paper wrapped in aluminum foil |
| 2 | sterile pipettes, 1 ml size |
| 4 | glass slides (1x3 inch) |
| 1 | pair forceps |
| 1 | beaker of alcohol for flame sterilizing forceps and glass slides |
| 1 | gloves |
| 1 | Overnight culture of Pseudomonas putida |
Instructions:
Constructing a Model Biofilm - Period 1
- Select one of the square petri plates containing Trypticase Soy Agar (TSA).
- Onto the surface of the agar, aseptically place one sheet of sterile filter paper. Do this by opening the aluminum foil package being careful not to touch the sterile filter paper sheets inside. Sterilize a pair of forceps by dipping them in a container of alcohol and flaming them. When the flames go out the forceps are sterile. Pick up one filter paper sheet and transfer it to the surface of the TS agar plate. Place the sheet so that no air bubbles are trapped under the filter paper. Trapped bubbles can be removed by pushing them with the sterile forceps to the edge of the filter paper.
- Dilute the overnight culture by pipetting 1 ml of the culture into 9 ml of 1/10 normal strength TSB. Thoroughly mix the culture by vortexing or by rolling the tube containing the culture between the palms of your hands.
- Uniformly moisten the filter paper with 1 ml of the diluted bacterial culture.
- Select a clean 1 X 3 inch microscope slide and using the forceps, immerse the slide in a container of alcohol and sterilize it by flaming. When the flame goes out the slide is sterile. Allow the slide to cool for a few seconds and then carefully lay the slide on top of the filter paper. Press the slide down with the forceps, being careful that no air bubbles are trapped beneath the slide.
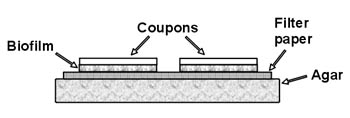
- Repeat step 5 three more times. If you are careful, you
should be able to place four 1” X 3” slides
side by side on the plate
(See Figure 1). - Between Periods 1 and 2 If possible, remoisten the plate after 24 hours with 1 ml of 1/10 strength TSB. Note: If scheduling problems make this impossible this step may be omitted, but more robust biofilms are produced if this is done.
Period 1
- After two days the biofilms may be harvested by carefully lifting the slides from the filter paper surface with a pair of sterile forceps (flamed). The biofilm should appear as a slimy layer coating the under surface of the slide. Lifting too rapidly may disturb the biofilm causing large sections to slough off the slide.
- Be sure to dispose of all materials according to your teacher’s instructions.
Illustration:
Reference
Charaf UK, Bakich SL, and Falbo DM, 1999, A Model Biofilm for Efficacy Assessment of Antimicrobials Versus Biofilm Bacteria,IN Biofilms: The Good, The Bad and the Ugly. J. Wimpenny, P Gilbert, J. Walker, M. Brading and R. Bayston, Eds.
Published by BioLine for the Biofilm Club, Cardiff University, UK, ISBN 0-9520432-6-2.
Educational Program Curricula and Teaching Resources
Supported in part by the Waksman Foundation for MicrobiologyDeveloped in collaboration with Dr. John Lennox, Penn State University-Altoona
©1999-2008 Center for Biofilm Engineering, http://www.biofilm.montana.edu
