Copyright © Alfred B. Cunningham, John E. Lennox, and Rockford J. Ross, Eds. 2001-2010
Biofilms as Biobarriers1
Student Version (go to Instructor Version)
Introduction:
Under certain circumstances, biofilms can be used to retard the spread of organic and inorganic contaminants in ground water by filling the pores of the soil with EPS (extracellular polymeric substances). The EPS fills the pore spaces of the soil and reduces the mass transport of water. In this exercise you will create a biofilm in a sand column and measure the rate of flow of water through the column as the biofilm grows.
Supplies Needed:
|
Quantity |
Description |
| 1 | sand column (See Figure 1) |
| 1 | overnight culture of Pseudomonas fluorescens (or other BSL-1 organism) |
| As Necessary | Tubes containing 20 ml aliquots of molasses medium |
| 1 | 150 ml beaker |
| 1 | sterile 25 ml pipette |
| 1 | stopwatch |
| As Necessary | gloves |
| 1 | container for the disposal of waste medium |
Protocol:
Safety note: During this exercise you should wear latex or other protective gloves. The column should be mounted securely in a ringstand and the entire apparatus should be placed on a tray to collect any spillage in the event of an accident.
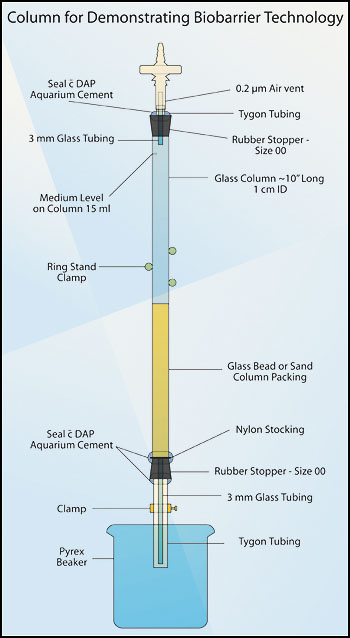
Figure 1. Column for demonstrating biobarrier
technology.
Period one:
- Obtain a culture of Pseudomonas fluorescens from your instructor. This is a soil bacterium noted for its production of EPS. It has been grown up in Molasses medium, the same medium that you will be using to “feed” the column. The sand in the column is intended to represent soil, a rather porous medium.
- Using a sterile 25 ml pipette, add enough of the culture at the top of the column to bring the medium to the level of the sand. At this point, all the pores in the sand column should be filled with culture.
- Using the same pipette, now add an additional 15 ml of the culture to the column. Carefully mark the 15 ml level on the column with a magic marker or a piece of lab tape.
- While holding the Tygon™ tubing at the base of the column closed, remove the clamp.
- Release the Tygon™ tubing and with a stopwatch, record the time taken for the culture to just reach the surface of the packing material. Record this value as the pre-biofilm drainage time. Re-attach the clamp and incubate the column at room temperature until the next laboratory period. Make sure the medium is just at or slightly above the packing material surface. If you miss recording the timing for any reason, not to worry, just add more medium to the 15 ml line and try again.
Period two:
- On the desk you will find tubes of sterile molasses medium. Add sterile molasses medium to the column to the 15 ml mark. As before, remove the clamp while holding the Tygon™ tubing closed. Release the tubing and record the time taken for the medium to just reach the surface of the packing material.
- Record the time it takes to drain 15 ml of medium in a table indicating time and number of days after inoculation of the column.
- Note any evidence of microbial growth in the pores of the column seen through the wall of the column.
- On a graph, plot drainage time vs day post inoculation in seconds. You maay find that recording ml/sec a more useful measure of biobarrier formation. (15 ml/drainage time in sec = ml/sec).
Subsequent lab periods:
- Continue to take readings on the flow rate of the column as long as any significant change is noted. One might pool class data and graph the results.
Questions:
- At the end of the exercise, how much (percentage) has the flow rate been reduced by the presence of the biofilm?
- Sometime during the growth of the biofilm in the column, the flow rate will temporarily increase. Based on your understanding of biofilm growth, can you account for this observation?
- Describe a scenario or write a news report in which this phenomenon might be used to reduce the effects of environmental contamination.
Reference
1Subsurface biofilm barriers for the containment and remediation of contaminated groundwaterCunningham AB, Sharp RR, Hiebert R, James G
Bioremediation Journal, 2003; 7:151-164
Educational Program Curricula and Teaching Resources
Developed in collaboration with Dr. John Lennox, Penn State University-Altoona©1999-2008 Center for Biofilm Engineering, http://www.biofilm.montana.edu
