Buccal Epithelial Cells with Adherent Bacterial Cells Revealed by Negative Staining
Student Version (go to Instructor Version)
Introduction
The human body represents an enormous and extraordinarily diverse collection of surface habitats and micro-niches potentially available for colonization by biofilm associated micro flora. At birth the body is essentially sterile but soon becomes colonized by a variety of microorganisms. With continuous exposure to environmental surfaces and to human contact the biofilms become increasingly complex and gradually approach those typical of adults for a given geographical and perhaps even ethnic population. This accumulation of microbial cells eventually out numbers the human cells in the body by as much as tenfold. Despite this huge population of microbes, much of the body remains sterile in healthy individuals. The blood, and most body fluids, the heart, skeleton and skeletal muscles and the tissues of most organs, brain, liver, kidneys, the lymphatic system and the body cavities remain sterile until exposed to serious illness or injury. Other body regions including the skin, the gastrointestinal tract, the urogenital tract and the oropharynx develop a rich and diverse array of biofilms. In many instances such as periodontal disease, organisms present are the same as those found in healthy mouth tissues. The difference lies in the proportion of organisms present and the time over which they can have their affect. Maintenance of the healthy micro-flora ratio depends upon proper diet (low in sugar), and proper oral hygiene. That is to say, your mother was right all along.
The flora of the mouth is one of the most intensively studied human microbial communities. Over 500 different species of bacteria have been identified in the oral cavity. Microbiologists have concentrated on the microbes that form plaque on the teeth because this flora is responsible for dental caries and periodontal disease (Kolenbrander and Palmer, 2004). Relatively few investigations of the buccal mucosa have been done and those pertain mostly to children (Wilson 2005).
Supplies Needed:
Quantity |
Description |
| 1 | tongue depressor |
| 2 | 1 x 3 inch glass microscope slides |
| As Necessary | Nigrosine dye |
| 1 | Oil immersion equipped microscope |
| As Necessary | Immersion oil |
Safety Note: tongue depressors should not be thrown in regular trash. They should be put either in an autoclave bag or in a beaker with bleach.
Students should wash their hands before leaving the lab.
Dispose of used glass slides in biohazard trash or bleach.
Instructions:
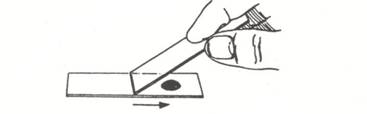
- Place a small drop of nigrosine dye near one end of a clean, standard (1 x 3 inch) microscope slide.
- Using a tongue depressor, gently scrape the inside of your cheek (buccal surface) so as to remove buccal epithelial cells.
- Pat the material gathered from the cheek into the drop of nigrosine dye.
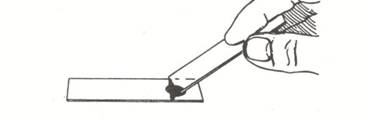
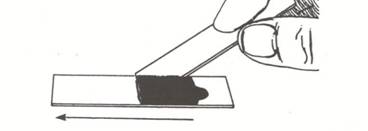
- Hold a second clean slide at about a 45 degree angle to the slide containing the nigrosine dye. Back the slide up until it touches the dye/epithelial cell mixture. You should see the dye spread from side to side on the slide. Drag the drop of dye down the slide until the dye runs out.
- Allow the negatively stained slide to air dry (do not heat fix) and examine the buccal cells under the oil immersion lens.
Illustration:
Observations and questions:
- Make a drawing of one squamous epithelial cell and include representative prokaryotic cells at the same scale. If you can, estimate the size of the epithelial cell and of the bacterial cells using a calibrated ocular micrometer. Use this information in the following calculation. Or adopt this alternative procedure using the squamous epithelial cell photo, Figure 2.
- You will immediately notice an enormous difference in size between a typical prokaryotic cell and a typical eukaryotic cell. Can you estimate the order of magnitude difference in volume between these prokaryotic cells and the epithelial cell?
- Using the scale bar and the assumption that the pictured squamous cell is rectangular and rather flat (thickness about 15 µm), estimate the volume of the epithelial cell.
- Using the scale bar, estimate the diameter of the streptococcal cells on the epithelial cell surface, and estimate the volume of a typical prokaryotic cell (πr3).
- Determine the ratio by division. What is the order of magnitude of the difference?
- Microbiologists have estimated that there are more prokaryotic cells in the human body than eukaryotic cells (Wilson, 2005). Count the number of prokaryotic cells adhering to 10 eukaryotic cells. What is the ratio of prokaryotic cells to eukaryotic cells in this human tissue?

Reference:
Wilson, M. 2005., Microbial Inhabitants of Humans: Their Ecology and Role in Health and Disease. Cambridge University Press. Cambridge, UK.This material is based upon work supported by the National Science Foundation under Grant No. 0618744, and in part by the Waksman Foundation for Microbiology. Developed in collaboration with Dr. John Lennox, Penn State Altoona. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
©2002-2008 Center for Biofilm Engineering, http://www.biofilm.montana.edu
