Emergent Properties of Biofilms
"It is a property of complex systems that they exhibit properties not predictable from the individual components of the system.” For Goldstein, emergence can be defined as: "the arising of novel and coherent structures, patterns and properties during the process of self-organization in complex systems." (Corning 2002)
Marsh and Bowden (2000) have pointed out that biofilms exhibit emergent properties, in that the properties of the biofilm community are greater than, and not predictable from, the known properties of the individual microbial components of the biofilm.
Perhaps the most dramatic of these is the development of the complex architecture characteristic of many pure culture and mixed culture biofilms. The organization of towers and mushrooms, streamers and water channels was totally unsuspected until investigators employed the new technology of confocal microscopy in examining biofilms. But beyond structural complexity, there are other properties, which follow as consequences of the intricate and multiple systems of intercellular communication, which have been discovered over the last five decades. These emergent properties include increased resistance to antimicrobials, virulence, interaction with eukaryotic host cells, and alternating reproductive strategies. The constituent components of biofilms may even be capable of altruism and “cheating”, properties usually attributed to much more sophisticated organisms.
These attributes share a common property, that is, they are all regulated by another emergent property—they all involve the production and response to small molecular weight signal molecules in a process called quorum sensing or cell-cell signaling.
These communication capabilities have led some to think of biofilms as proto-multicellular organisms with division of labor and functional specialization (Shapiro, 1998).
Cell-Cell Signaling
When first discovered in bioluminescent bacteria by Nealson and Hastings (1977) the concept of autoinduction was thought to be a quaint and unique feature of a few marine organisms. Today it is recognized that homologues of the system that controls light production in Vibrio fischeri and V. harveyi are to be found in many, perhaps most bacteria. They have been found to control an enormous variety of functions related to population density including antibiotic production, biofilm production, virulence, tumor formation by Agrobacterium tumefaciens, swarming motility in Proteus spp., competence in Streptococcus pneumoniae, sporulation in Bacillus spp. and inter-species communication in a wide variety of unrelated microbial taxons (glossary).
In its simplest conception, autoinduction, or quorum sensing as it is now called, is a mechanism by which bacteria can prevent the transcription of certain gene clusters until a minimal critical population density has been achieved. This is accomplished through the production of low molecular weight, highly diffusible, compounds (autoinducers) which, in V. fischeri, are made by an enzyme which in turn is the product of the Lux I gene.
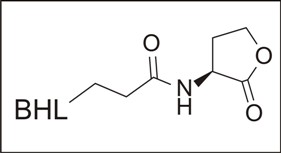
Figure 1. An Acyl-Homoserine Lactone.
These compounds, (chemically acyl homoserine lactones, see figure 1) are produced continuously by the bacterium and they diffuse across the plasma membrane and out into the surrounding environment. In the open ocean, or pond or blood stream, these compounds are rapidly diluted and their intercellular concentration remains low. When, however, a bacterium attaches to a surface in the early steps of biofilm formation, half the universe into which these molecules might have diffused is eliminated and autoinducer concentration begins to rise. As the cell divides and its descendants begin to enclose themselves in an EPS matrix, the concentration of these compounds increases even more. At some point the concentration becomes sufficient (micromolar concentrations) so that they bind allosterically to an RNA polymerase enzyme that, is the product of the Lux R gene.
When activated by acylhomoserine lactone, this polymerase transcribes the rest of the genes in the Lux operon including the gene for the enzyme luciferase which is responsible for the oxidation of the long chain adehyde that produces bioluminescence (Figure 2).
Since 1977, similar QS systems have been found in many bacterial systems including Gram positive bacteria which typically use amino acids and oligo-peptides rather than AHSL molecules as their autoinducers. These autoinducer molecules represent a language among bacteria, which permits conservation of energy when population density is not high enough for a particular function to be effective. It is now known that many bacteria engaging in cell-cell signaling produce inducers that members of other species respond to. Such important messages as “keep the water channels open” are “obeyed” not only by the bacterium producing the message but also by bacteria of other species, which also benefit by the nutrients and oxygen that the open water channels deliver. Thus bacteria not only communicate, they are multi lingual using a secondary Quorum Sensing system mediated by an autoinducer called AI2 (See Figure 3) (Bassler, 2001).
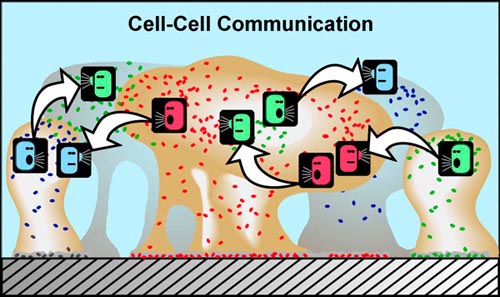
Figure 4. Cell-Cell Communication.
Complex Architecture
A long-standing goal of biofilm research as been to find the factors which influence the formation of the complex structures produced by many bacterial species. Researchers have explored physical factors (sheer, surface composition), and physiological factors (nutrient concentration, species composition, oxidation reduction potential) in search of the key, which would solve the puzzle. With the discovery of acyl homoserine lactones in gram negative bacteria (Fuqua et al. 1994) and the role of quorum sensing in many biofilm related activities (virulence, swarming, bioluminescence) it was only natural to ask if this system also played a role in biofilm structure. This question was answered, for Pseudomonas aeruginosa at least, in an elegant experiment carried out by Davies and his collaborators (Davies et al. 1998). The wild type of this organism produces tower and mushroom-like microcolonies when growing on surfaces. It had been earlier demonstrated that the las I gene was responsible for the production of 3OC12-acyl homoserine lactone (AHL). This autoinducer binds to a specific RNA polymerase which inturn transcribes a number genes related to virulence, attachment and other biofilm related functions. Davies demonstrated that a mutant of the lasI gene, incapable of making the AHL produced biofilms that were only about 20% as thick as that made by the wild type organism. In addition these biofilms were produced as continuous horizontal flat sheets rather than the vertical towers expected from this organism. Furthermore, with the addition of exogenous AHL to the medium in which the las I mutant was growing, the biofilm reverted to a structure indistinguishable from that produced by the wild type strain. This indicates that 3OC12-acyl homoserine lactone, is at least in part , responsible for the complex architecture of P. aeruginosa biofilms.



