What is the connection between biofilms and persistent infections?
Biofilms tolerate antimicrobial agents
One of the hallmarks of the biofilm mode of growth is reduced susceptibility to all types of antimicrobial agents. Microorganisms in biofilms are protected from antimicrobials across the spectrum of modes of action, from oxidants such as chlorine and hydrogen peroxide to antibiotics with exquisitely specific targets. Antibiotic tolerance of bacteria in biofilms is easily reproduced in vitro, showing that host factors are not required to manifest the biofilm defense. The protective mechanisms at work in biofilms appear to be distinct from those that are responsible for conventional antibiotic resistance. In biofilms, poor antibiotic penetration, nutrient limitation and slow growth, adaptive stress responses, and formation of persister cells are hypothesized to constitute a multi-layered defense. The poor efficacy of antimicrobials against biofilm embedded bacteria likely contributes to the inability of antibiotics or antimicrobial components of the host defenses to resolve some persistent infections. It has been well demonstrated that bacteria within biofilms are hundreds to thousands of times more resistant to antibiotics and biocides than their free floating (planktonic) forms. Recent research has suggested that biofilm bacteria are phenotypically distinct from their planktonic counterparts. Many of the bacterial genes involved in biofilm formation are controlled by the same regulatory systems that control virulence factors.
Biofilm chemistry and antimicrobial susceptibility
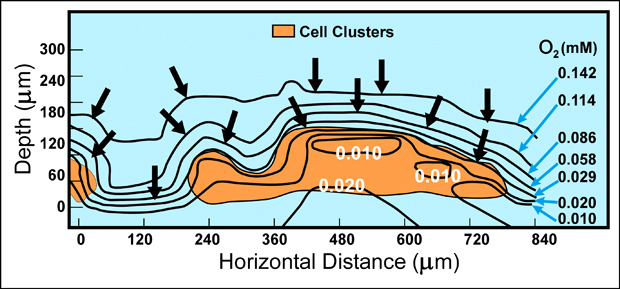
Microscale variations in the chemical environment within biofilms explain many of the phenomena that are characteristic of these communities. Direct measurements of chemical conditions in individual microcolonies, using needle-shaped microelectrodes with tips only 10 microns across, have shown that parameters such as pH and dissolved oxygen concentrations vary radically between locations as close as 50 microns apart in the biofilm community (Figure 7-2). As these chemical parameters vary, so does the physiological status and ecology of the microbial community. For example, in a biofilm composed solely of the obligately aerobic bacterium Pseudomonas aeruginosa, the zone of oxygen penetration and zones of respiration and growth coincide and are limited to the outer 20 to 30 microns of the biofilm (Xu et al, 1998).
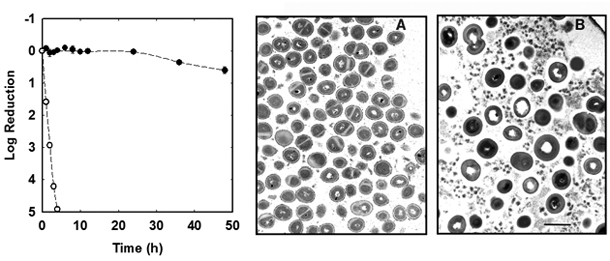
The Stewart laboratory has years of practical experience in measuring the antimicrobial susceptibility of planktonic and biofilm bacteria (Anderl et al, 2000; Anderl et al, 2004; Borriello et al, 2004; Cochran et al, 2000; Hassett et al, 1999; Huang et al, 1996; Mah et al, 2003; Vrany et al, 1997; Walters et al, 2003; Zheng and Stewart, 2004). An example using an in vitro grown staphylococcal biofilm is shown in Figure 7.3.